Zackeryeh Salloum – MRes Student, University of Portsmouth
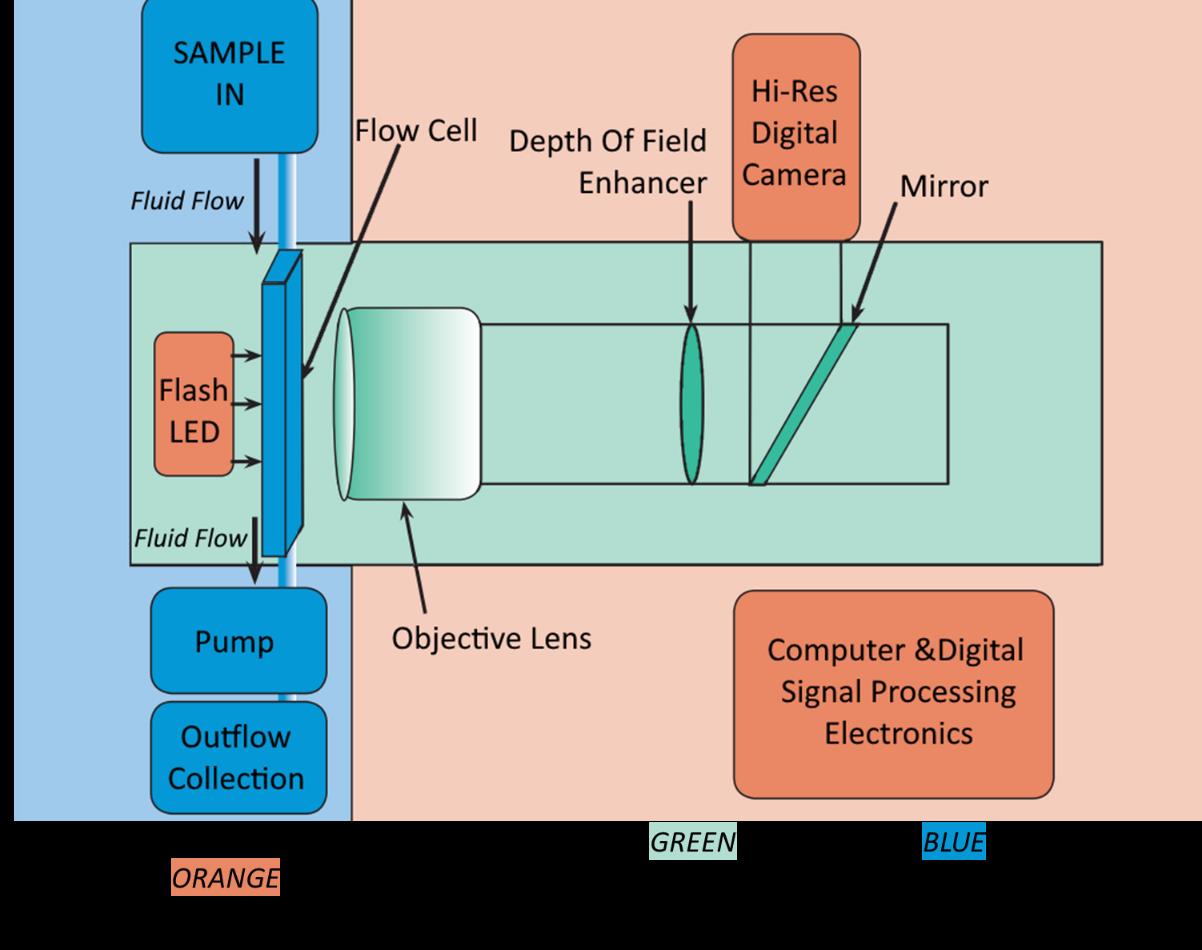
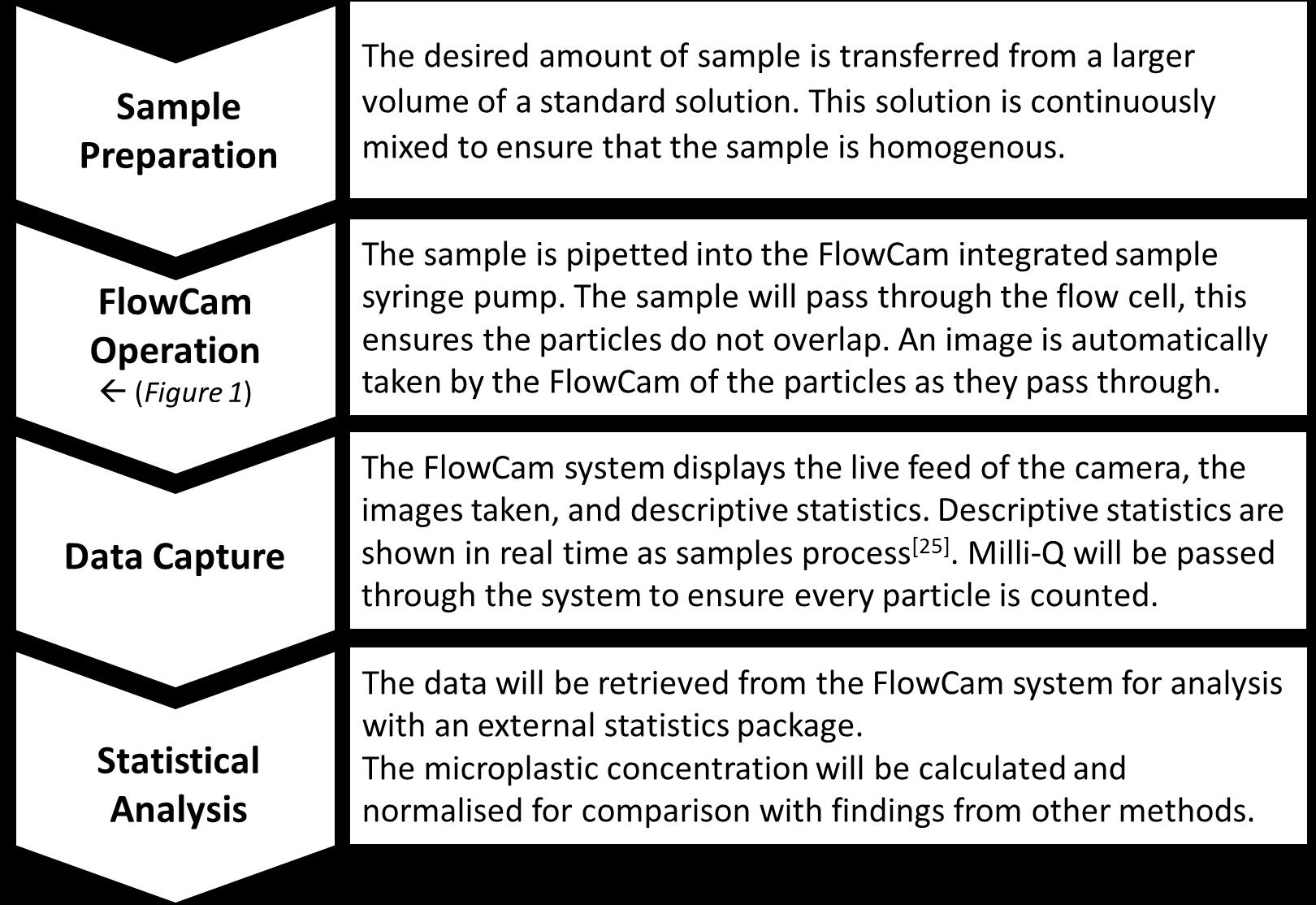
We have awarded a grant to Zackeryeh Salloum, an MRes stduent working in the School of Environment, Geography and Geosciences in Portsmouth. Zack previously studied Bsc Marine Environmental Science at the University of Portsmouth and completed his final year project on the changing microplastic distribution within Swansea Bay in two varying seasons. This included the use of multiple lab methods in microplastic analysis and identification, from which the further research is being developed. This research will make up the main body of an MRes project, the aim is to publish the final report and develop a new method of microplastics analysis.